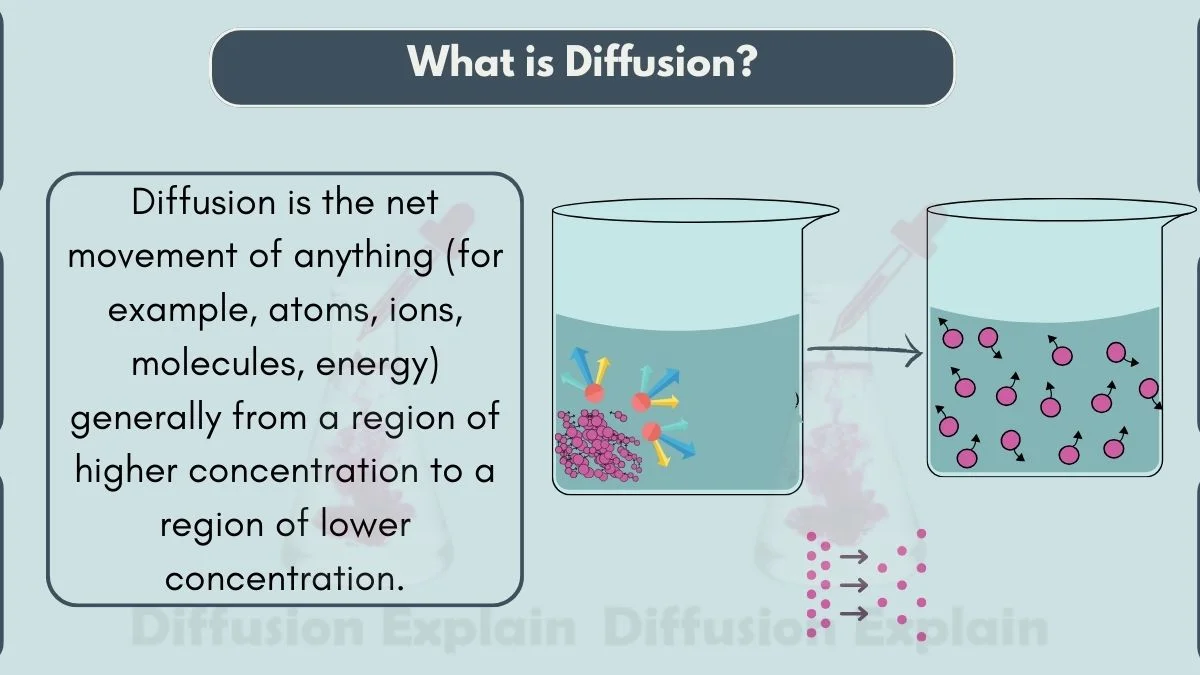
Diffusion Definition
In science, diffusion is the process of evenly distributing dissolved particles from an area where they are more concentrated to an area where they are less focused. This diffusion meaning explains how particles move and spread out due to their natural, random motion. Diffusion requires no extra energy because particles already possess thermal energy that pushes them to move.
In simple words, what does diffuse mean in science? It means to spread out until evenly mixed. This is why diffusions occur in gases, liquids, and even solids when particles have enough space to move. So, what is the process of intermixing of particles called? It is called diffusion.
1: What best explains diffusion?
Diffusion is best explained as the passive movement of particles from an area of high concentration to low concentration, because particles are always in random motion and spread out to reach equilibrium.
2: What is an example of a diffusion process?
An example is oxygen moving from the lungs into the blood, because the oxygen concentration is higher in the alveoli than in the blood, and particles naturally move down their concentration gradient.
What is Diffusion?
What is diffusion in practical terms? It is the natural movement of particles from a region where they are crowded to a region where they are fewer. What is the process of diffusion? It begins when there is a difference in concentration and continues until particles become evenly spread. This is the process of evenly distributing dissolved particles, and it does not need any outside force.
Diffusion helps smells spread through the air, sugar mixes in water, nutrients move into cells, and heat shifts from warmer to cooler regions. The main idea is simple: diffusion is the spontaneous intermixing and spreading of particles until the concentration becomes equal everywhere.
Understanding what is diffusion makes it clear why this concept is central to chemistry, biology, and everyday processes.
Which direction do molecules naturally diffuse?
A) From low to high concentration
B) From high to low concentration
C) Randomly with no net movement
D) Towards colder areas only
Answer: B
In simple diffusion, molecules move from an area of ____ concentration to an area of ____ concentraetion.
Answer: high; low
Diffusion Meaning Across Biology, Chemistry, Physics, Physiology, and Anatomy
Diffusion is the same basic idea in all subjects: particles move from crowded areas to less crowded areas. Here’s how it matters in different subjects: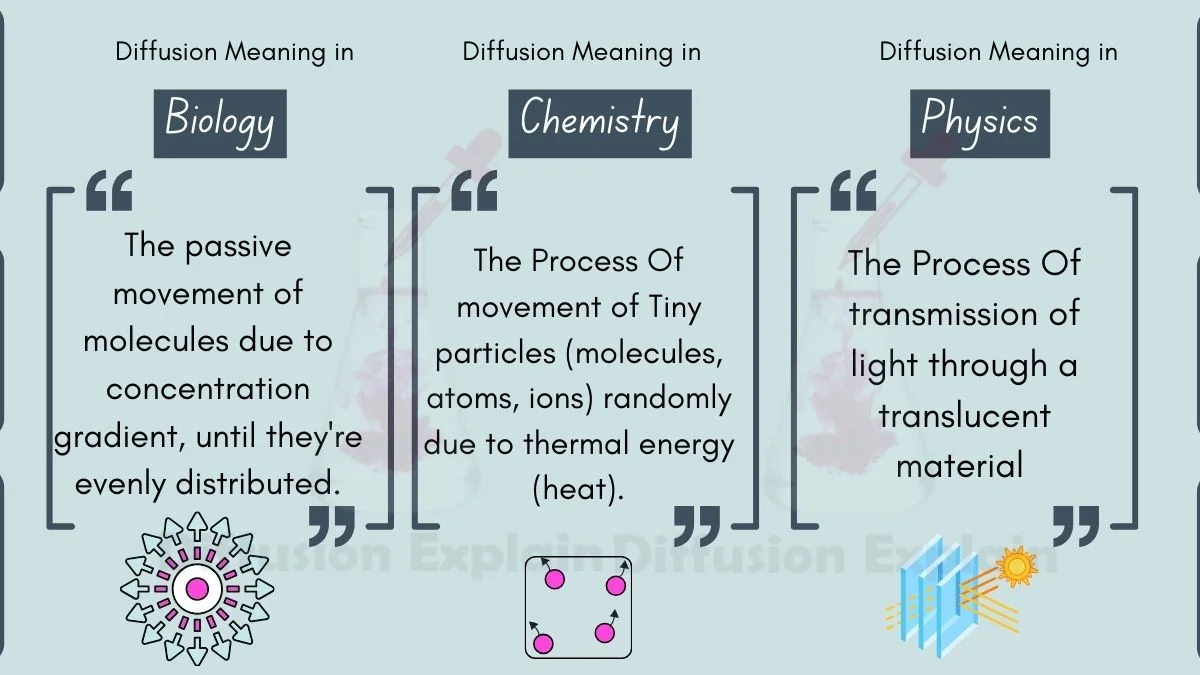
|
Subject |
How Diffusion Works |
Why It Matters |
|
Biology |
Gases like oxygen and carbon dioxide, or nutrients, move in and out of cells. |
Helps cells get what they need and remove waste. |
|
Chemistry |
Molecules or ions spread out in liquids, gases, or solids. |
Explains how substances mix and reactions happen. |
|
Physics |
Tiny particles move randomly and spread out over time. |
Shows how energy and motion cause particles to mix naturally. |
|
Physiology |
Oxygen and nutrients diffuse from blood to tissues; waste diffuses back to blood. |
Supports life by delivering nutrients and removing waste efficiently. |
|
Anatomy |
Diffusion happens across membranes and tissues, like lungs, intestines, and kidneys. |
Explains how body structures are designed for efficient exchange of gases, nutrients, and wastes. |
Diffusion in Biology
In biology, diffusion is essential for survival because it controls how substances enter and leave cells. The direction of diffusion always goes from higher concentration to lower concentration, creating a net movement of particles. This net movement determines whether substances move into the cell, out of it, or remain balanced.
What is required for diffusion to occur? Two conditions must exist:
- A concentration gradient
- A membrane that is permeable to the molecule
Without these, what is necessary for diffusion to take place does not exist, and movement will not happen.
A key fact: diffusion never occurs against concentration gradients. Moving substances against the gradient requires active transport, which uses energy. This clarification prevents confusion between passive and active processes.
True or False: Diffusion takes place only in liquids.
Answer: False
A common misunderstanding is that diffusion occurs against concentration gradients. It does not. Biological diffusion is a form of passive transport and never occurs against concentration gradients. Movement against the gradient requires energy and is known as active transport. This distinction prevents confusion between passive and energy-dependent processes.
So, do molecules stop moving when diffusion stops? No. When equilibrium is reached, net diffusion stops, but molecules never stop moving. They continue to move randomly, but movement in both directions becomes equal.
Where is diffusion found in the body?
Diffusion is found throughout the body, for example in the lungs, where oxygen moves into the blood, and in cells, where nutrients and waste products are exchanged, because these processes rely on particles moving from high to low concentration.
What is diffusion in plants?
Diffusion in plants occurs when gases like carbon dioxide enter leaves for photosynthesis and oxygen exits, because the gas concentrations inside and outside the leaf differ. That’s why plants can exchange gases without using energy.
Does diffusion change with age?
Yes, diffusion can change with age because cellular efficiency and membrane permeability may decrease, which affects how quickly substances move across membranes. That’s why older tissues sometimes exchange gases and nutrients more slowly.
Specialized Biological Examples
Diffusion in Alveoli (Lungs)
The alveoli are tiny air sacs with thin walls, a large surface area, and a dense capillary network. These adaptations maximize the rate of gas diffusion. Oxygen diffuses from the alveoli (high concentration) into the blood (low concentration), while carbon dioxide diffuses in the opposite direction. The structure of alveoli perfectly aligns with diffusion requirements, speeding up gas exchange.
Diffusion in Villi and Microvilli (Small Intestine)
Villi and microvilli increase the intestinal surface area tremendously. This vast surface allows nutrients such as glucose and amino acids to diffuse efficiently from the gut into the bloodstream. Their thin epithelial lining and rich blood supply ensure rapid absorption due to diffusion.
These examples highlight diffusion facts and show how nature designs structures to maximize the movement of particles along the concentration gradient.
Where can diffusion occur?
A) Only in liquids
B) Only in gases
C) Gases, liquids, and some solids
D) Only in solids
Answer: C
Diffusion in Chemistry
What is diffusion in chemistry? It is the movement of particles in liquids, gases, and solids, driven by their natural motion toward lower concentration. Thus, the idea that diffusion takes place only in liquids is false. This broader perspective explains why diffusion controls reactions, corrosion, and mixing processes in materials.
Key Types of Diffusion in Chemistry and Materials Science
Types of diffusion in chemistry include:
- Molecular Diffusion – movement due to random molecular motion
- Knudsen Diffusion – occurs when pore size is smaller than particle mean free path
- Configurational Diffusion – movement in very narrow pores or channels
These types of diffusion in chemistry help scientists understand how substances spread in different environments.
Which of the following explains the mechanism of diffusion?
A) Particles move from low to high concentration using energy
B) Particles move randomly and spread from high to low concentration
C) Molecules remain stationary
D) None of the above
Answer: B
Diffusion in Physics
Diffusion meaning in physics refers to the spread of particles due to random motion. In this context, diffusion is studied in terms of energy, probability, and particle motion. In physics: It is the movement of particles from regions of higher density to lower density due to random motion.
The meaning of diffusion in physics is closely tied to Brownian motion, discovered by Robert Brown in 1827. Robert Brown observed that small particles suspended in water move irregularly (Brownian motion). Later, Einstein explained that this motion is caused by molecules colliding with the particles. This phenomenon demonstrates the meaning of diffusion in physics and is the basis for understanding random mixing of gases and fluids.
This phenomenon became known as Brownian Movement, which explains what is the random mixing of gas molecules called, it is precisely this random molecular motion.
Albert Einstein later provided the mathematical explanation for Brownian motion, proving the existence of atoms and molecules through their random diffusion patterns.
Principle of Diffusion
Diffusion explains why particles spread out on their own. They don’t need a push, a machine, or any special force. They move because of their own energy, and this movement continues until everything becomes evenly mixed.
1. Movement From High To Low Concentration
Diffusion happens when there is a difference in the number of particles between two places. This difference is called a concentration gradient.
Particles naturally move from an area where they are crowded (high concentration) to an area where there are fewer particles (low concentration). They keep moving like this until both areas have almost the same number of particles.
That concentration gradient answers two questions clearly:
- Why does diffusion occur? Because particles spread out to balance concentration.
- What is required for diffusion to occur? A concentration gradient—without this difference, nothing will move.
A simple real-life example: When salt dissolves in water, the salt particles spread throughout the water from the area where salt is dropped (high concentration) to all parts of the solution (low concentration). No one stirs, yet the particles move and mix.
Diffusion occurs because:
A) Molecules are static
B) Molecules move randomly and seek equilibrium
C) Molecules are heavy
D) Molecules attract each other
Answer: B
2. Driven by random motion
Particles don’t travel in straight lines, and no one tells them where to go. They move all the time due to their own kinetic energy, the energy they have because they’re constantly in motion. This explains:
- What causes diffusion? The inherent kinetic energy of particles.
- What drives diffusion? Thermal energy, which keeps particles moving randomly.
- How do molecules move during diffusion? By bumping into each other and changing direction again and again.
This random, continuous movement is known as Brownian motion. Over time, this motion spreads particles out evenly, which describes the movement of molecules in diffusion without needing any external help.
Diffusion works because particles are energetic and never stay still. They move randomly, and this motion pushes them from crowded regions to less crowded ones until everything is equal.
Cause-and-Mechanism of Diffusion
|
Question |
Answers |
|
Why does diffusion occur? |
Because particles have thermal energy, and that energy makes them move randomly. |
|
What causes diffusion? |
The kinetic energy inside molecules, because it keeps them in constant motion. |
|
What drives diffusion? |
Thermal energy, because it powers the random movement known as Brownian motion. |
|
How do molecules move during diffusion? |
They move randomly because collisions make them change direction again and again. |
|
What is required for diffusion to occur? |
A concentration gradient, because particles move from crowded to less crowded areas. |
Does net diffusion eventually come to an end?
Yes, net diffusion eventually comes to an end because the concentrations on both sides become equal, and there is no longer a driving force for movement. That’s why equilibrium is reached.
When diffusion stops, it has reached?
When diffusion stops, it has reached equilibrium, because particles are evenly distributed and there is no net movement.
What is the end of diffusion?
The end of diffusion is equilibrium, because diffusion continues until there is no concentration difference remaining.
What would stop diffusion from occurring?
Diffusion would stop if there is no concentration gradient or if particles cannot move freely, because these are the conditions required for particles to spread from high to low concentration.
Types of Diffusion
There are three main types of diffusion found in living systems.
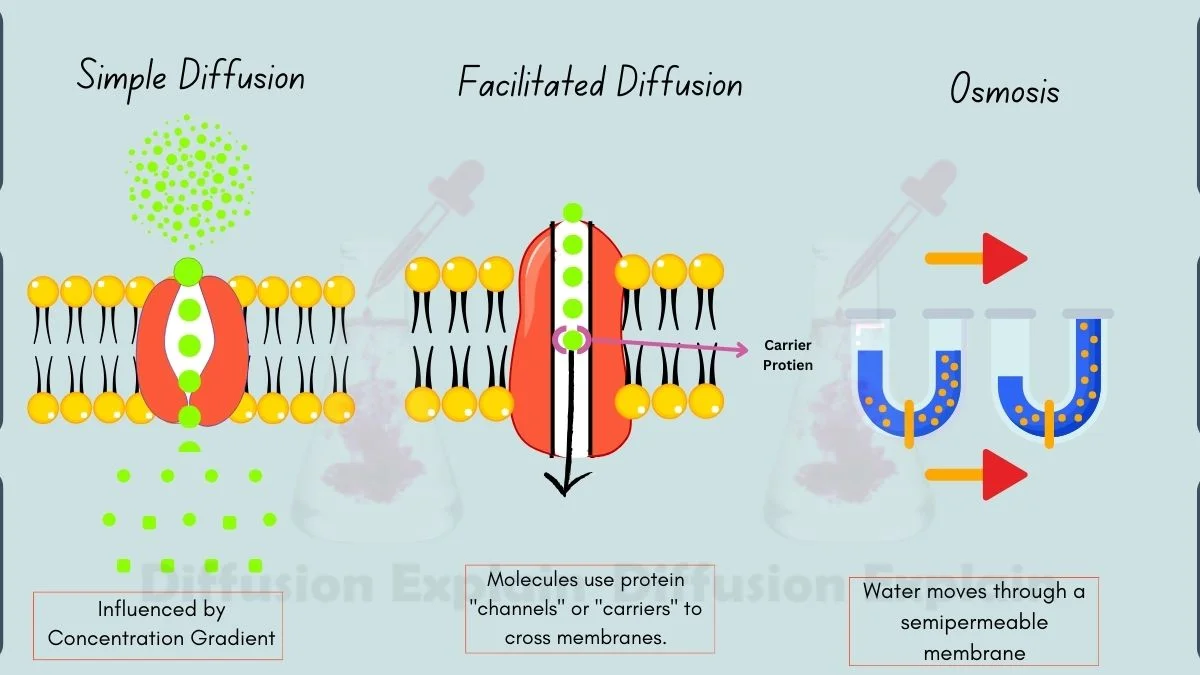
1. Simple Diffusion
Simple diffusion occurs when small, non-polar molecules move directly across a permeable membrane from an area of high concentration to an area of low concentration without using cellular energy. Gases like oxygen (O₂) and carbon dioxide (CO₂) cross the lipid bilayer this way because they can dissolve in the membrane’s hydrophobic core.
The speed of simple diffusion depends on the concentration gradient, membrane permeability, and the size of the molecules involved. This process is essential for exchanging gases during breathing.
Example
Oxygen diffuses from the air inside the lungs into the blood. Since its concentration is higher in the lungs than in the bloodstream, the net movement of oxygen is inward.
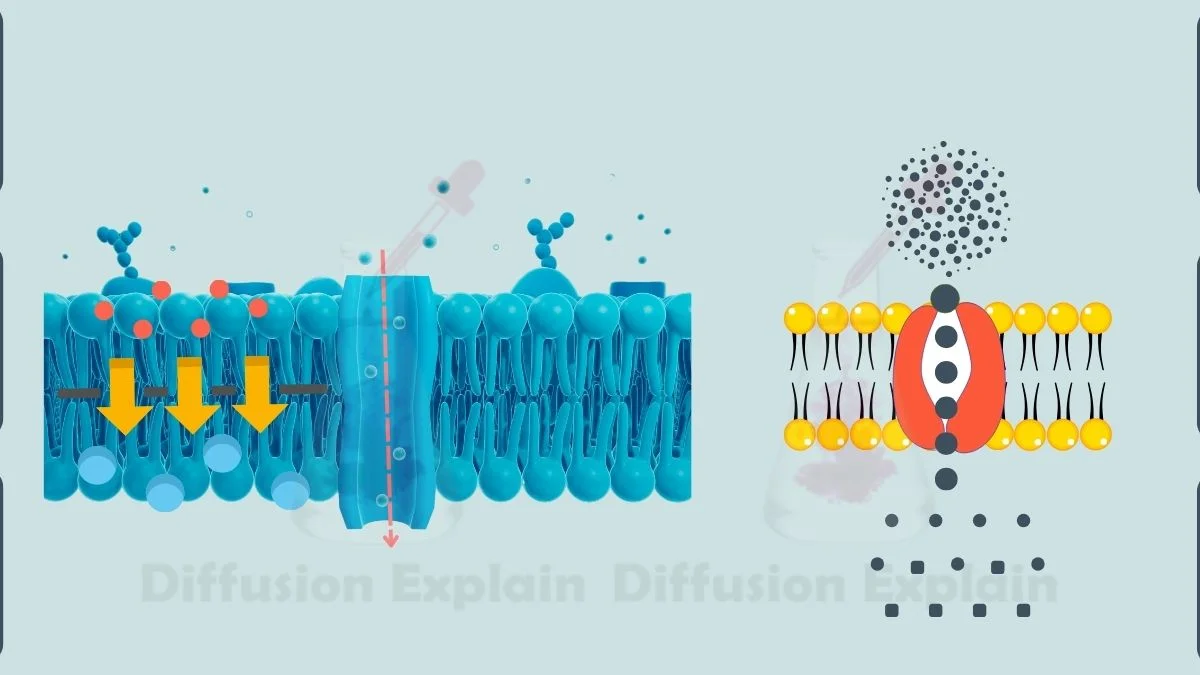
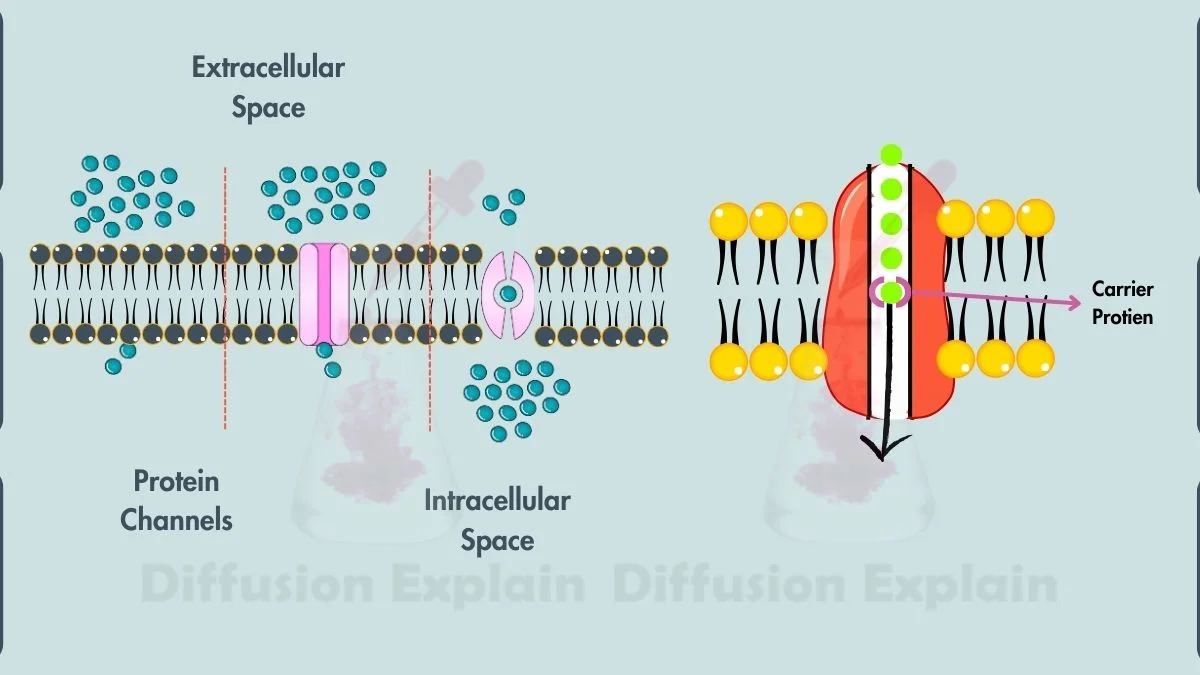
2. Facilitated Diffusion
Facilitated diffusion also moves particles down a concentration gradient, but only with the help of transport proteins. These channel or carrier proteins allow larger or charged molecules such as glucose and amino acids, to cross the membrane, which they cannot do through the hydrophobic lipid bilayer alone.
This mechanism regulates what enters and leaves a cell, ensuring only specific molecules pass through.
Example
Glucose enters muscle cells through a protein channel designed to recognize and move it. The glucose molecules still move randomly, but the channel makes their transport faster and more controlled.
3. Osmosis
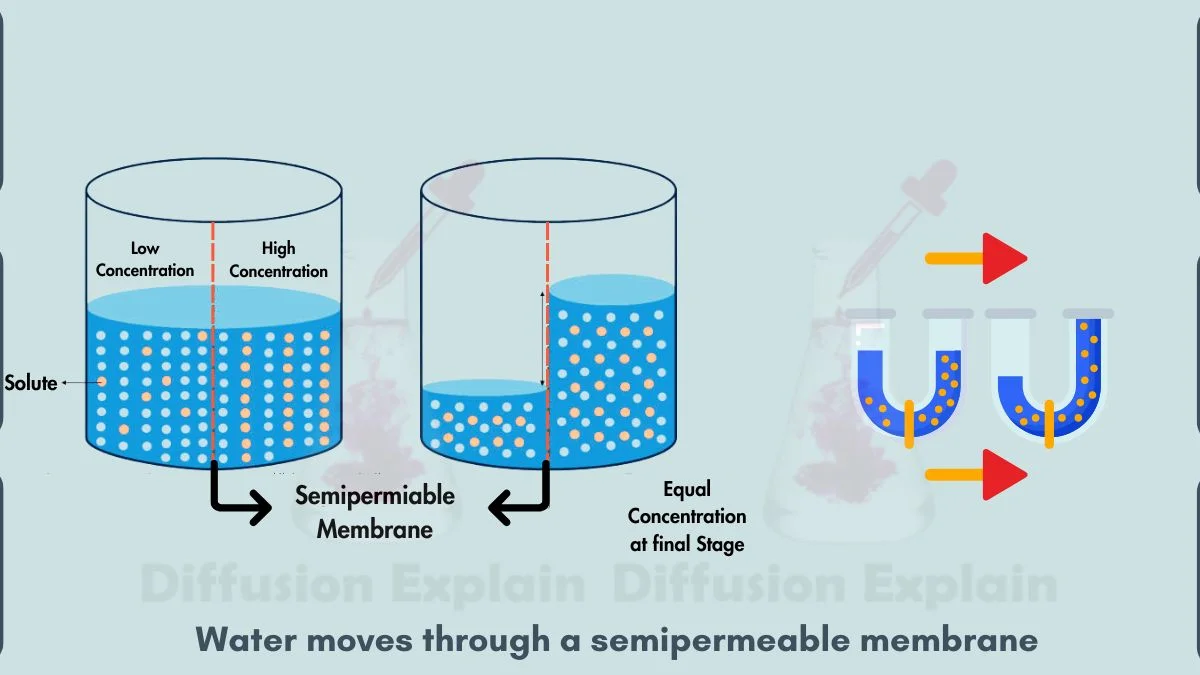
Osmosis is the diffusion of water through a selectively permeable membrane. Water moves from an area with more water (low solute concentration) to an area with less water (high solute concentration). The difference in solute levels creates a pull that draws water across.
Osmosis maintains hydration and pressure inside cells. Without it, plant cells would wilt and animal cells could not regulate fluids properly.
While both are related processes, there are important differences between diffusion and osmosis in terms of molecules transported and driving forces.
Example
Water enters a plant cell because the inside of the cell contains more dissolved substances than its surroundings. The net movement of water is toward the higher solute area.
While the above three types are passive processes requiring no energy, cells also use active transport mechanisms that consume ATP to move molecules against their concentration gradients.
Common Diffusion Experiments
Here are some simple experiments to observe diffusion in action. Each experiment shows how particles move randomly from an area of higher concentration to an area of lower concentration in a medium, until they are evenly spread (equilibrium), without needing any extra energy.
1. Food Coloring in Water
Fill a clear container with water and add a drop of food coloring. Watch how the color slowly spreads through the water in a food coloring diffusion experiment. The dye molecules move randomly through the liquid medium, traveling from high concentration (the drop) to lower concentration areas until the water is uniformly colored.
2. Tea Bag Diffusion in Hot Water
Place a tea bag in a cup of hot water. Over a few minutes, you will see the water color gradually spreading from the tea bag into the rest of the cup, demonstrating tea bag diffusion in action. The tea molecules move randomly through the water medium from high concentration (inside the tea bag) to lower concentration areas until the color is evenly distributed.
3. Potato Osmosis
Cut a potato into small cubes and place them in two containers of water. Add salt to one container, leave the other plain. After some time, the potato cubes in saltwater shrink while those in plain water stay the same. This shows osmosis, a type of diffusion where water moves through a semi-permeable membrane, from areas of low solute concentration (inside the potato) to high solute concentration (saltwater), until concentrations balance.
4. Perfume in a Jar
Place a cotton ball soaked with perfume or essential oil at the bottom of a jar. Seal the jar and wait. The scent spreads as perfume molecules move randomly through the air medium, from high concentration near the cotton ball to lower concentration areas until evenly distributed through perfume diffusion.
5. Sugar Cube in Water
Drop a sugar cube into a glass of water. Over time, the sugar dissolves and spreads through the water. Sugar molecules move randomly through the liquid medium from high concentration (the cube) to lower concentration areas, until the water is evenly sweetened.
Diffusion Experiments Overview
|
Experiment |
Medium |
Core Mechanism |
Observation / Outcome |
|
Tea Bag Diffusion in Hot Water |
Liquid |
Net movement of tea molecules, passive transport |
Color spreads from the tea bag into the water until uniform (equilibrium) |
|
Perfume Diffusion in Air |
Gas |
Random kinetic motion of perfume molecules |
Scent spreads throughout the room, molecules move from high to low concentration |
|
Food Coloring Diffusion in Water |
Liquid |
Net movement of dye molecules, passive transport |
Dye spreads until water is uniformly colored, showing movement along a concentration gradient |
Factors Affecting Rate of Diffusion
Here are some factors that affect the rate of Diffusion
Concentration Gradient
The concentration gradient provides the main driving force for diffusion by creating uneven distribution that molecules then spread to correct. Areas with high concentrations have frequent molecular collisions, pushing molecules toward adjacent areas of lower concentration where fewer particles occupy the space. The difference in particle density across the diffusion interface creates an imbalance that pulls materials from high to low concentration.
The difference in how many molecules or ions there are in one place compared to another determines how fast things move around. If there’s a big difference in concentration between two areas, things will move faster from the crowded place to the less crowded one. But as the difference in concentration gets smaller, the movement slows down until everything is spread out evenly. This movement from crowded to less crowded areas happens faster when the difference in concentration is bigger. Once everything is evenly spread out, diffusion stops.
Temperature
Higher temperatures impart greater kinetic energy to molecules, increasing their random molecular motion and velocity. The more energetic and rapid particle movement results in faster molecular diffusion between collisions. Increased collisions from faster particles speed contact across the concentration interface. Thermal energy excites particles to overcome resistance when changing direction. Raising systems temperatures decreases fluid viscosity as another diffusion enhancement. Therefore, hotter systems exhibit accelerated diffusion rates.
Concentration
A higher absolute concentration means more particles occupying the same volume or area. With greater numbers of molecules packed within closer proximity, the frequency of encounters and collisions is increased. At interfaces with lower concentrations, a higher number of molecules spread from a dense area allows more particles to come into diffusive contact and transition spaces simultaneously. Abundant concentrated particles have numerous opportunities to randomly disperse. Maximum concentrations create molecular crowding that drives rapid diffusion into less concentrated regions.
Diffusion Distance
The distance over which molecules must traverse gradients determines duration before particles reach remote areas. With lengthy distances, molecules require more time and individual steps for Brownian walks to cover long expanses between origin and destination of diffusion. Each step along the way delays equilibration as gradients dissipate over time into widespread regions. Short diffusion lengths enable rapid transit and mingling of neighboring zones. Longer travel requirements mean particles take extensive steps before uniformly populating the space, reducing diffusion velocity over distance.
Surface Area
The expanded surface area provides more contact interface through which molecules can transfer and interact between concentration disparities. Broader interfaces allow simultaneous diffusion of many molecules rather than queued single file across narrow passages that limit the number of particles diffusing concurrently.
With vast aspects open for transit, abundant molecules speed flux rather than waiting at slimmer membranes that restrain flow amounts. Wider barriers enable carefree passage as molecules rapidly mingle by utilizing available space. Confined surfaces severely limit molecular exchange and delay diffusion toward equilibrium.
Molecular Size
Small molecules move easily because they’re tiny and light. They can zip through without much trouble, sliding between other molecules with little resistance. They take quick breaks between bumps, which helps them move faster overall. But big molecules move slower. They’re like big trucks trying to squeeze through traffic. They face more resistance and move more slowly through liquids or cell membranes. Their size slows down their movement compared to smaller molecules.
Describe two variables that affect the rate of diffusion
The rate of diffusion is affected by temperature, because higher temperatures increase particle movement, and concentration gradient, because a greater difference in concentration drives faster diffusion.
What are the variables of diffusion?
The main variables of diffusion are temperature, concentration gradient, diffusion distance, surface area, and particle size, because each factor influences how quickly particles can move from one area to another.
Which factor does NOT affect the rate of diffusion?
A) Temperature
B) Particle size
C) Concentration gradient
D) Color of the molecules
Answer: D
Diffusion in Liquids
In liquids, diffusion occurs as particles randomly move and spread out, transitioning between frequent molecular collisions from regions of high to low concentration. The particle mobility in liquids enables relatively quick diffusion, though particle size and viscosity impact diffusion rates.
Adding a soluble dye to water is an example of diffusion in liquids. The color disperses as dye molecules diffuse through the solvent until evenly distributed. Ion mobility in electrolytic liquids also demonstrates diffusion through concentration gradients.
Diffusion in Solids
Within rigid closely packed solid structures, atoms and molecules have restricted mobility, leading to very slow solid-state diffusion. Over long time periods, material transport happens from high to low-concentration areas as fluctuations allow particles to incrementally exchange positions. This allows the diffusion of new alloying elements through metals and semiconductors during fabrication. Tight atomic spacing creates resistance, yet defects like vacancies and interstitials facilitate gradual solid diffusion.
Diffusion in Gases
Gaseous particles have high kinetic energies and large mean free paths between collisions, moving rapidly to fill any volume. When concentration differences exist, gas molecules quickly diffuse across distance gradients, rapidly spreading from high to low-density areas. For example, air freshener scent particles rapidly disperse throughout a room based on random motion until evenly distributed. Gaseous state and low viscosity minimize resistance enabling great diffusion Mobility even over longer distances compared to liquid or solid states.
Applications of Diffusion
Here are some important applications of diffusion:
- Oxygen diffuses into cells, while carbon dioxide diffuses out, enabling energy production.
- Diffusion facilitates the uptake of essential nutrients across cell membranes.
- Neurotransmitters diffuse across synapses, carrying signals between neurons.
- Diffusion helps eliminate waste products from tissues.
- Drugs can passively diffuse across membranes, delivering their therapeutic effects.
- Gaseous uranium isotopes are separated through diffusion, crucial for nuclear power.
- Diffusion blends polymers and additives to create materials with desired properties.
- Pigments and dyes diffuse into fabrics for vibrant colors.
- Diffusion controls the distribution of solutes during crystal formation.
- Diffusion helps create alloys by distributing different metal atoms.
- Diffusion rates can reveal damage to cell membranes due to disease.
- This life-saving technique uses diffusion to filter blood in kidney failure patients.
- Diffusion-based sensors can detect toxic chemical leaks.
- Drugs can diffuse through the skin for localized treatment.
- Diffusion models predict the movement of greenhouse gases between different Earth systems.
- Diffusion transports heat from the Earth’s core towards the surface.
- Diffusion helps understand how contaminants move through groundwater.
- Diffusion plays a role in mixing sediments and dissolved minerals in water bodies.
FAQs
1. What is diffusion?
Diffusion is the movement of molecules or particles from high concentration to low concentration until they are evenly distributed, because particles naturally spread out to balance differences.
2. Why does diffusion occur?
Diffusion occurs because molecules are in constant random motion, and they move from regions of higher concentration to lower concentration to reach equilibrium. This is why diffusion is a spontaneous process.
3. Why does diffusion happen?
Diffusion happens due to the random motion of particles and the concentration gradient. Particles naturally spread out to balance concentration differences, because systems always move toward equilibrium.
4. What causes diffusion?
Diffusion is caused by molecular motion and differences in concentration, because particles move from high to low concentration until equilibrium is achieved.
5. How does diffusion occur?
Diffusion occurs when particles move randomly and collide, spreading from high concentration to low concentration, because the concentration difference drives the net movement.
6. How do molecules move during diffusion?
Molecules move in all directions randomly, but the net movement is from high to low concentration because particles follow the concentration gradient.
7. Where does diffusion occur?
Diffusion occurs in gases, liquids, and solids, wherever particles are free to move and a concentration gradient exists, because these conditions allow particles to spread naturally.
8. When does diffusion occur?
Diffusion occurs whenever there is a concentration difference between two regions and particles can move freely, because this difference provides the driving force for movement.
9. What does it mean for a molecule to diffuse down a concentration gradient?
It means the molecule moves from an area of higher concentration to lower concentration, following the natural slope of the concentration gradient, because particles always move toward equilibrium.
10. What determines the direction of diffusion?
The direction of diffusion is determined by the concentration gradient, because particles move from high to low concentration to reduce differences.
11. Do molecules stop moving when diffusion stops?
No. Molecules continue random motion, but net diffusion stops once equilibrium is reached, because there is no longer a concentration difference driving movement.
